The principle of sodium thiosulphate standardization is based on redox titration utilizing the iodometric method in which potassium bromate is used as the oxidizing agent. Potassium Iodate Solution Standardization.

Doc Standardization Of Sodium Thiosulphate Na2s2o3 Solution With A Potassium Dichromate K2cr2o7 Solution Meharaj Mahmmud Academia Edu
The 250 ml primary standard solution is prepared by a precisely weighed amount of the potassium bromate is dissolved in its water.

. This method is developed using an automated titration system Excellence Titrator. Potassium iodate reacts with excess KI in acid solution according to the following reaction. Take 100 mL distilled water in conical flask Add 2g of Potassium di-iodide Add 10 mL of potassium di chromate And lastly add 2.
This can be titrated with a standardized sodium thiosulfate solution to form a clear solution. Then titrate with the sodium thiosulphate until most of the liberated iodine has reacted and the. To 200 ml of this solution add 2 g of potassium iodide and 10 ml of 1M sulphuric acid.
Sodium thiosulfate sodium thiosulphate is an inorganic compound with the formula Na 2 S 2 O 3. A 01 M sodium thiosulphate. Sides of the flask with 200 mL water and titrate with your thiosulfate solution until the brown iodide color becomes noticeably lighter.
Immediately add 5 mL starch indicator and continue to. The principle of standardization of sodium thiosulphate is based on redox iodometric titration with potassium iodate as primary standard. Introduction Sample Preparation and Procedures Chemistry Instruments and Accessories Method in Detail.
So if the titration is not performed immediately then there could be an error in determination of strengt. Enter concentration and volume of the sample click Use button. Iodine solution with a small amount of starch forms a dark blue solution.
Then the iodine concetration in the original solhtion can be calculated. Add 25 grams of potassium iodide and 4-5 mL of 4N hydrochloric acid to this solution. Three sample of 07 09 g of solid KHP are place into each of the three numbered Erlenmeyer flasks.
Download thiosulfate standardization against iodine reaction file open it with the free trial version of the stoichiometry calculator. The iodine liberation process significantly affects the titration results. Chemical and reagent preparation is very crucial for any test.
Sign in and order today. Uniformity of reactions between iodine and sodium thiosulphate forms. Sign in and order today.
Make up a standard solution of potassium iodate by accurately weighing by difference about 01 g of KIO3 and placing it in your 100 mL volumetric flask. 5 rows Procedure. Take 248 g of sodium thiosulphate Na2O3S2 and dissolve in 200 ml of distilled water in a volumetric flask and properly mixing it.
Click nCV button over iodine. To standardise 0025 N sodium thiosulfate. The mixture is shaken until the salts dissolve then conc.
Sodium thiosulfate is often standardized with potassium dichromate. Clean the washed burette with the approximately 01 mol dm-3 standardised sodium thiosulphate which is going to be prepared. We must prepare chemicals and reagents to get the accurate test results.
Hydrochloric acid is added. In this process first we need to liberate iodine to react with sodium thiosulfate. Potassium iodate a strong oxidizing agent is treated with excess potassium iodide in acidic media which liberates iodine which is back titrated with sodium thiosulphate Procedure.
The first section is to standardize the Sodium Hydroxide by titration. But sodium thiosulfate is commonly used in iodometry and iodimetry so I am assuming you are using Na 2 S 2 O 3 for that purpose. In the standardization iodine triiodide liberated by potassium dichromate in an acidic potassium iodide solution is titrated with a sodium thiosulfate solution.
Standardization of Sodium Thiosulphate Solution Pipette 25 mL of the standard potassium bromate solution into a 250 mL Erlenmeyer flask. Once it has completely dissolved make up the volume to 1000 ml. Fill the flask to mark with distilled water.
Standardisation of 01N sodium thiosulphate Take 10 ml of Potassium Iodate solution Add 2 gm of Potassium Iodide and 5 ml of dilute H 2 SO 4keep it in dark for 10 minutes add 2 to 3 drops of starch indicator andtitrate with sodium thiosulphte using starch solution as indicator until the blue colour is disappeared. Firstly wash out a burette twice with distilled water. Standardization of sodium thiosulfate using potassium dichromate.
The principle of standardization of sodium thiosulphate is based on redox iodometric titration with potassium iodate or potassium bromate as a primary standard. Sodium thiosulfate is used in gold mining water treatment analytical chemistry the. Ad Complete your research with 300000 products.
In that case iodine is sensitive to light and decomposes back to iodide in the presence of light. Potassium iodate a strong oxidizing agent is treated with excess potassium iodide in acidic media which liberates iodine which is back titrated with sodium thioslphate. Standardization of the Sodium Thiosulfate Solution.
ASTM D1510-16 also describes the standardization method for sodium thiosulfate solution using KIO 3 KI as a primary standard. Dissolve 25 g of sodium thiosulphate and 02 g of. Preparation of 01 N potassium iodate.
Click nCV button below thiosulfate in the output frame enter volume of the solution used read solution concentration. Dilute 250 ml of the solution to 100 ml with water. Sodium thiosulphate solution can also used to reduce iodine to iodide.
First sodium bicarbonate is added to a iodate-free solution of potassium iodide. X H 2 O. Typically it is available as the white or colorless pentahydrate Na 2 S 2 O 3 5H 2 OThe solid is an efflorescent loses water readily crystalline substance that dissolves well in water.
Ad Complete your research with 300000 products. 50 ml of distilled water are added to each three of it from graduated cylinder and constantly shake it until the KHP solution are completely Premium Sodium hydroxide Titration. Titrate with 01 M sodium thiosulphate using 1 ml of starch solution added.

0 005 M Sodium Thiosulfate Standard Solution Dilute 50 Cm3
![]()
Standardization Of Sodium Thiosulfate By 0 1 N Potassium Dichromate Solutions Docsity

How To Prepare Standardization 0 1 N Sodium Hydroxide Naoh Sodium Hydroxide Sodium Preparation

Pdf E Dcapodaca Chm112l Preparation And Standardization Of A Sodium Thiosulfate Solution Ma Juryst Chelsea Armas Academia Edu

How To Prepare Standardization 0 1 N Potassium Dichromate

Sodium Thiosulfate New Hope For The Treatment Of Calciphylaxis Hayden 2010 Seminars In Dialysis Wiley Online Library

Standardisation Of Sodium Thiosulphate 0 02 Molar Solution Of Sodium Thiosulphate Youtube

0 1 N Sodium Thiosulfate Preparation And Standardization

Standard Preparation Chemistry Experiments Life Science Chemical Reactions

How To Prepare Standardization 0 1 N Potassium Permanganate Colored Glass Bottles Glass Containers Potassium

Chrominfo Preparation And Standardization Of 0 1 N Sodium Thiosulphate
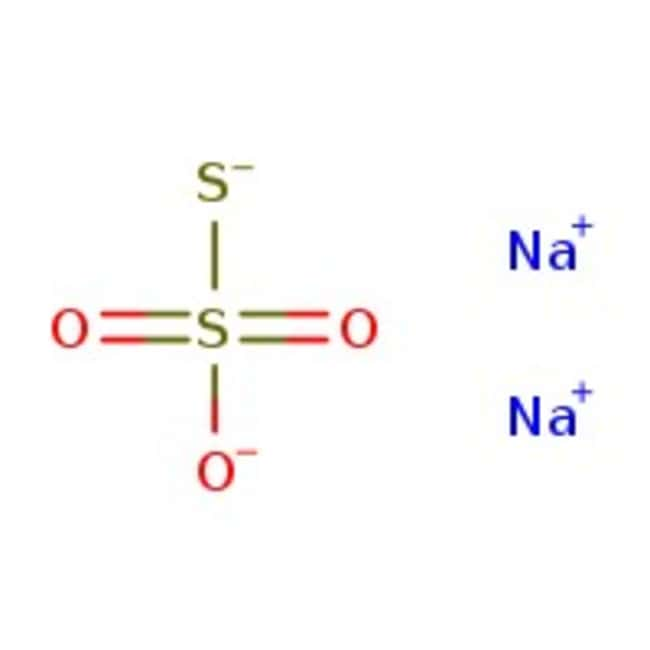
Sodium Thiosulfate 1 0n Standardized Solution Thermo Scientific

0 05 M Edta Solution Preparation Standardization
Standardize Sodium Thiosulfate According To Astm D1510 16

Standardization Of Sodium Thiosulphate Solution With Standard
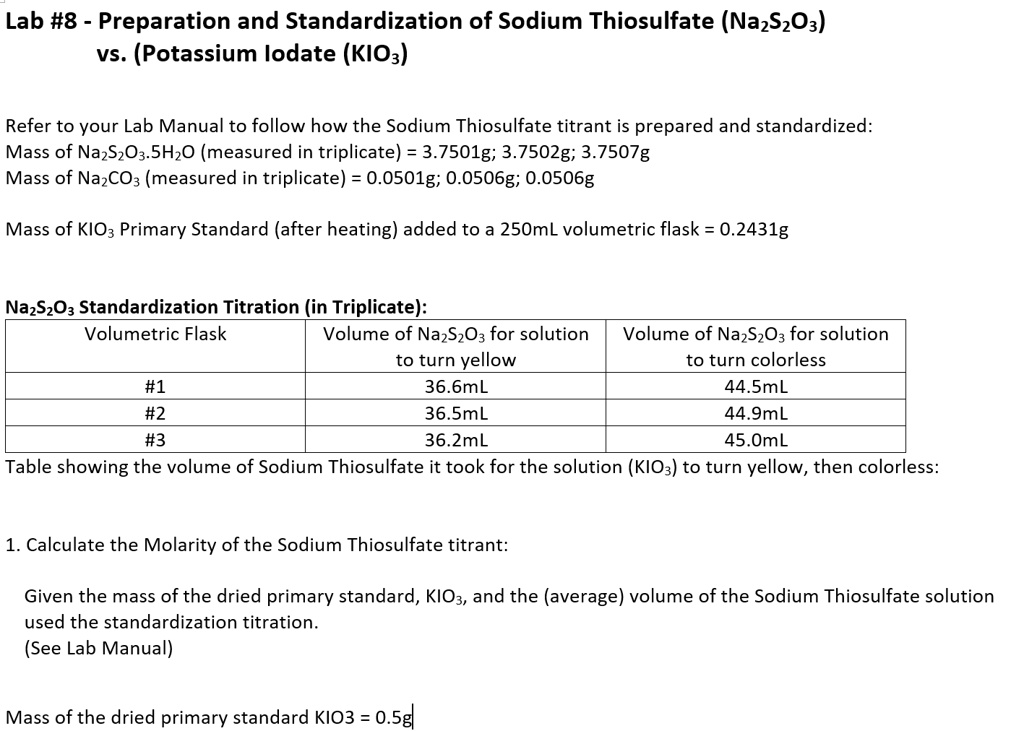
Lab 8 Preparation And Standardization Of Sodium Thios Itprospt

Preparation And Standardization Of Sodium Thiosulfate By Generose Carumba


